Inherited genetic abnormalities can raise a person’s chances of getting cancer, especially gynecological cancers. Many forms of cancer have the common cause of losing the ability to regulate the development of cells and fix damaged DNA.
Genetic variations in the regulation of cell growth and DNA repair are especially likely to be linked to an overall elevated risk of cancer. It leads to the emergence of different cancers bearing identical genetic alterations and overlapping clinical presentations of multiple syndromic cancers. Many of the previously identified hereditary cancer syndromes are caused by genetic abnormalities.
Genetic testing can determine whether an inherited syndrome causes a disorder, identify the causal genetic variation(s), assess the cancer risk of a patient’s blood relatives, and enhance results.
The Otogenetics Comprehensive Panel focuses on the genes that are shared by several hereditary cancer types. Next Generation Sequencing, a cutting-edge genetic testing tool, has the potential to provide the most comprehensive coverage and identification of genetic variants linked to cancer and cancer risk.
Cancer gene panels have the advantage of lowering the cost and increasing the effectiveness of cancer genetic testing by reducing the amount of time, patient visits, and tests sent. A negative genetic test is more reassuring at eliminating the likelihood of inherited risk when all known genes for that phenotype have been assayed.
A BRCA1 or BRCA2 mutation causes around 15% of all ovarian cancers.
A further 5-6% of ovarian cancers are caused by mutations in other genes, including the Lynch syndrome genes.
(BRIP1, RAD51D, RAD51C, PALB2, BARD1, TP53, MLH1, MSH2, MSH6, PMS2).
In fact, Otogenetics’ data from over 40,000 hereditary cancer gene tests supports the roles played by BRCA1 and BRCA2 but also reveals that other genes account for over 80% of positive instances. When using multi-gene testing, 40–50% more people with hereditary cancer gene mutations can be found than when using BRCA1 and BRCA2 testing alone. The simultaneous examination of numerous genes is a very cost-effective and time-efficient solution. Furthermore, certain health insurance plans only cover genetic testing once in a lifetime for a certain disease.
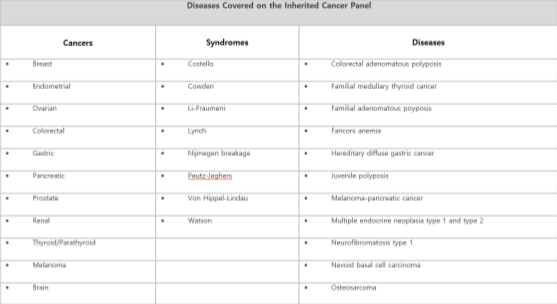
Otogenetics GxVision Hereditary Cancer Risk Assessment Test OptionsComprehensive Inherited Cancer Gene Tests – 45 genes
linked to Breast, Ovarian, Endometrial, Colorectal, Lynch Syndrome, Gastric, Melanoma, Pancreatic, Polyposis, Prostate, Renal, Thyroid/Parathyroid, Uterine and other major cancers
A hereditary cancer risk assessment is essential for identifying patients and families who may be at an elevated risk of getting specific types of cancer.
If a hereditary cancer risk assessment indicates a higher likelihood of a hereditary cancer syndrome. An appointment with a cancer genetics specialist or a genetics-trained healthcare provider is recommended for a more thorough review of family history data, risk evaluation, education, and counseling, which could result in genetic testing.
Mutations in the BRCA1 and BRCA2 genes produce hereditary breast-ovarian cancer (HBOC) syndrome, which is distinguished by a higher risk of early-onset breast, numerous breast primaries, male breast, epithelial ovarian, Fallopian tube, or primary peritoneal cancers.
Individuals with HBOC syndrome are more likely to develop pancreatic cancer, prostate cancer, or melanoma. If a hereditary cancer risk assessment indicates a higher likelihood of a hereditary cancer syndrome. A referral to a cancer genetics specialist or a genetics-trained healthcare provider is recommended for a more thorough review of family history, risk evaluation, education, and counseling, which could result in genetic testing.
An estimated 1 in 300 people have BRCA1 mutations overall. The frequency of BRCA2 mutations is estimated to be one in 800. The prevalence of mutations is higher in populations with founder mutations, such as Ashkenazi Jews, Icelandic people, and Mexican Hispanic people.
Genetic testing should be considered for appropriate high-risk individuals whose medical management will be influenced by the tested individual and/or their at-risk family members.
Depending on the family structure, there are differences in the likelihood of detecting a mutation using these criteria.
The likelihood of finding a familial mutation may be underestimated among people with little or no family history, such as fewer than two female first- or second-degree ancestors in either lineage who have lived past 45 years.
Families with a high number of unaffected female ancestors may have very low odds of finding a mutation.
Patients who have received an allogeneic bone marrow transplant should not undergo molecular genetic testing using current blood sample technology due to incorrect test findings caused by donor DNA contamination. One can utilize DNA that has been isolated from a fibroblast culture. If a buccal sample is not available, it should be explored with a knowledge of the possibility of donor DNA contamination.
Variant categorization is based on Mendelian principles and follows the five-tier classification scheme proposed by the American College of Medical Genetics, or ACMGG.
This finding indicates that a genetic alteration or mutation that raises a person’s lifetime risk of acquiring a certain cancer has been found. This variation increases the risk of developing cancer directly.
Some pathogenic variations may not be completely penetrative. A single pathogenic mutation might not be enough to produce disease on its own in the case of recessive diseases. Additional data is not expected to change the classification of this variation.
The final report offers thorough risk information for the mutation(s). This result does not imply that the patient has cancer or will acquire cancer in their lifetime. It is advised to follow up with doctors, healthcare providers, and genetic counselors.
The results indicate that a mutation or genetic change that is likely to enhance the lifetime risk of acquiring specific cancers has been identified. But there isn’t enough data available at this time to draw firm conclusions from the science.
The final report includes extensive risk information relevant to the mutation discovered. The patient does not necessarily have cancer or will get cancer at some point in their lifetime based on this finding. It is advised to follow up with doctors, healthcare providers, and genetic counselors.
Variant(s) of Uncertain Significance (VUS) are variations that do not fit into the pathogenic, presumably pathogenic, or benign categories according to ACMGG and/or other relevant professional standards, or for which the pathogenic, likely infectious, and benign criteria are conflicting. Currently, there is insufficient or conflicting information to provide a more precise classification of this variant’s cancer risk.
According to the outcome, there were no variants of unknown significance (VUS), likely pathogenic (LP), or pathogenic (Ph). The report does not include likely benign or benign variants; however, they are accessible upon request.
©Copyright 2024 Houston Diagnostics Lab. All Rights Reserved.